
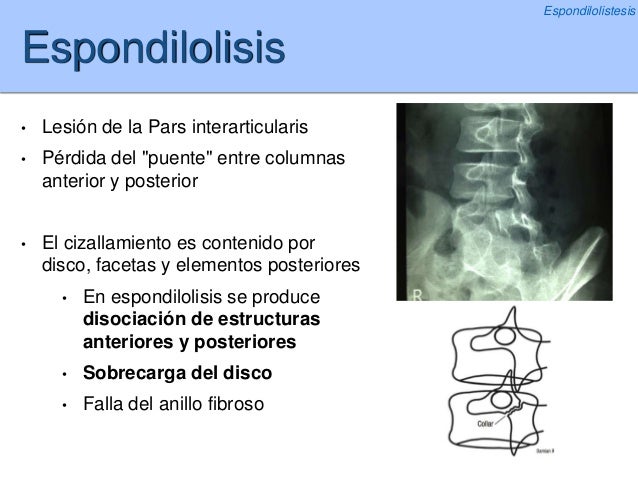
The complexity of the p62 interaction hub is remarkable and not easy to envision to function by simple one-to-one binding of protein interaction partners. Due to the multitude of discovered interaction partners, the p62 interaction hub is able to integrate the signals of multiple pathways such as selective autophagy ], MAP kinase ], NF-κB ], mTORC1 ], Nrf2 ], and N-end degradation ], linking p62 to many essential biological processes, such as degradation, oxidative stress, nutrient sensing, and inflammation. p62's UBA domain is critical for the recognition of poly-ubiquitin and ubiquitinated cargo ]. In addition, it contains a long intrinsically disordered region (IDR) (168–388), which provides binding sites to various interacting partners such as LC3, KEAP1, and FIP200 via short binding motifs ]. The p62 molecule is a 440 aa protein that contains three structurally folded domains: a PB1 domain (1–102), a ZZ-domain (122–167), and a UBA ubiquitin-binding domain (389–434). This so-called autophagosome is directed to the lysosome where it fuses with the lysosomal membrane and its contents are degraded. In this process, a phagophore membrane is recruited through p62's interaction with LC3 and elongated until it encloses the cargo-p62 complex in a double-membrane vesicle. Due to its early discovery, p62 can be considered the archetypical autophagy receptor that is also involved in the targeted removal of other cargo types such as bacteria, viruses, and organelles ]. The molecular link to autophagy was established by p62's involvement in the disposal of poly-ubiquitinated aggregates via the lysosome ].
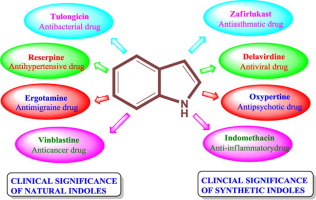
Further interaction studies revealed that p62 bridges the interaction of atypical protein kinase C (PKC) with RIP1 to activate the NF-κB pathway ]. Approximately 25 years ago, p62 was identified as a novel interaction partner of the SH2 domain of tyrosine–protein kinase Lck and subsequently cloned ].
#FRAMEWORK PROTEIN SCAFFOLD SERIES#
P62/SQSTM1 (from here on p62) is a multidomain, multifunctional protein involved in autophagy and a series of signaling processes ]. fluorescence recovery after photobleaching.To our knowledge, this is the first report regarding the application of MOFs as cell culture scaffolds and will serve as a starting point for studying two- and three-dimensional MOF-based cellular scaffolds for cell culture systems and for in vitro and in vivo tissue engineering. Importantly, C2C12 cells cultured on serum protein-preadsorbed fMIL-53 (Al) exhibited excellent long-term adhesion, morphology, and proliferation even in a medium lacking serum proteins, demonstrating an important advantage of fMIL-53 (Al) as a cell culture scaffold, given that conventional cell culture scaffolds typically require a serum-containing medium to support stable cell adhesion and proliferation. The viability of mouse myoblast cells (C2C12) cultured on fMIL-53 (Al) was 100%, indicating the cell compatibility of fMIL-53 (Al). β-Galactosidase, used as a model protein, adsorbed on fMIL-53 (Al) exhibited original enzymatic activity, indicating that proteins are not denatured during the adsorption process. The developed two-dimensional MIL-53 (Al) film exhibited high serum protein adsorption, retention, and replenishment capabilities as compared to conventional cell culture scaffolds. We therefore established a bottom-up technique to construct two-dimensional MOFs on polymer films. However, MOF nanoparticles cannot be used as two-dimensional scaffolds for cells. Several research groups recently reported that specific MOF nanoparticles can adsorb and retain proteins, suggesting to us that MOF nanoparticles may have advantages as novel cell culture scaffolds. MOFs are thus used in biomedical applications, and MOF nanoparticles have been widely studied as nanocarriers for drug delivery systems. Metal–organic frameworks (MOFs) are porous materials with adsorption, storage, and separation capabilities due to their high specific surface areas and large pore volumes.


 0 kommentar(er)
0 kommentar(er)
